Application Procedure IBBC
INFECTIOUS AGENT/MATERIAL APPLICATION PROCEDURE
OVERVIEW
Overview of the application procedure
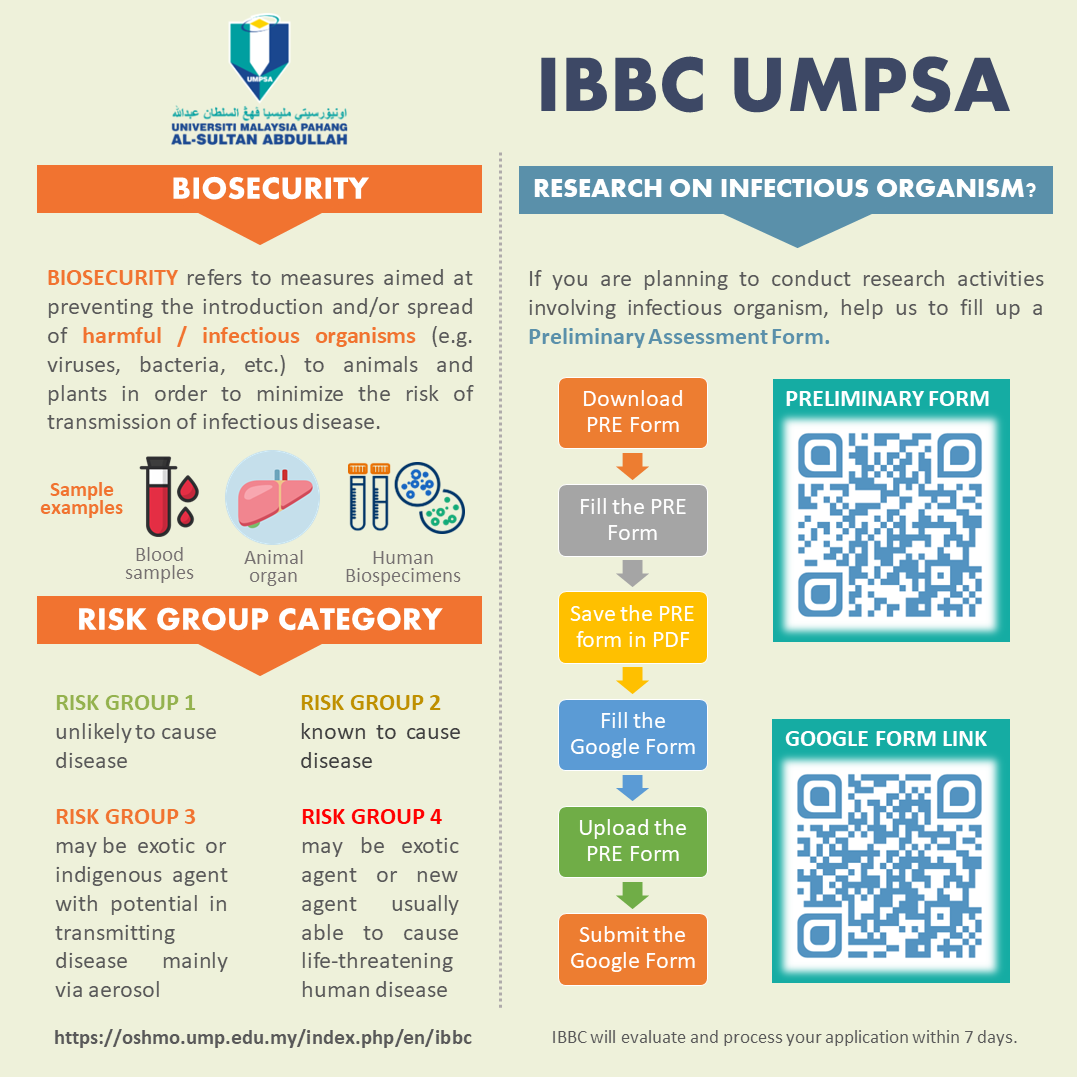
The applicant must obtain approval from the IBBC before the initiation of any activities that involve the handling, manipulating, working, using, storing, transporting and disposing of infectious and potentially infectious agents/materials and biological toxins. All applicants advised to firstly read and understand UMPSA policy and procedure prior to applying. A flowchart describing the overall IBBC approval procedure been included below. Prior to deciding on the need to perform an extensive application using the Form B (NOI) all applicants initially required to complete Form A (Preliminary Assessment Form). The information within this form will facilitate in the decision making of IBBC on whether the applicant should or should not complete Form B (NOI). Applicant has been provided with a guide on all possible infectious agents/material (List of Bio Risk Group) classified into risk group 1 – 4. Any infectious organism not found in the list or unknown to the applicant should be considered harmful and rightfully determined on its characteristic and pathogenicity. Additionally, verified with a consultation with the Expert Committee on Prevention and Control of Infectious Disease, Ministry of Health, Malaysia.
Flowchart (click here)
Resources
1. Malaysia Laboratory Biosafety & Biosecurity Policy and Guidelines (url)
References
1. UMPSA Policy and Guideline (click here)
2. List of Bio Risk Group (click here)
3. Final Version On Allowable Infectious Material in UMP (click here)
A. PRELIMINARY ASSESSMENT FORM (FORM A)
Applicant is advised to fill Form A also known as Preliminary Assessment Form. This form must be sent to the IBBC secretary to be evaluated and decide for progression either to complete form NOI (Form B) or exemption. The evaluation of Form A will take estimated 7 working days to complete.
Form A (click here)
List of Bio Risk Group (click here)
List of allowable infectious material in UMP (click here)
Please send your application to:
https://docs.google.com/forms/d/e/1FAIpQLSfBuyndtivKetbCMJWd8vByQFRNRoYN8vD9WcPvlcKyCr33pg/viewform
This process estimated to complete within 7 working days
B. NOTICE OF INTENT (FORM B)
Completed Notice of Intent (NOI) application must include all of these:
1. Submission Checklist (click here)
2. Notice of Intent (NOI) form (click here)
3. Biological Risk Assessment Form (Annex 1) (click here) (for exemplar referral purposes click here)
4. Laboratory Self-Inspection from Biosafety Level 1/2/3 Checklist (Annex 2 / Annex 3 / Annex 4) (select only one)
5. Personnel Biosecurity Registration Form (Annex 5) (completed in multiple copies if more than a single operator)
Please send your application to
This process estimated to complete within 14 working days
C. OTHER FORMS
1. Form C – Amendment from (click here)
2. Form D – Extension form (click here)
3. Annex 6 – Material transfer agreement (click here)
INFOGRAPHIC FOR PRELIMINARY ASSESSMENT FORM